Summary
Chronic pain in the lumbosacral spine (e.g. an L3–L5 spondylosis with nerve compression) can trigger full-body compensatory behaviors aimed at pain relief. These include pacing, bracing against doorframes, sitting on shower floors, continuous postural shifting, and other instinctive adjustments. Such behaviors are somatic compensations – the body’s way of alleviating nerve pressure or modulating pain signals – yet they are often misinterpreted as signs of psychological dysfunction by observers or even clinicians. This paper introduces the concept of Somatic Artifact Mapping, which recognizes environmental imprints (worn surfaces, improvised supports, assistive tweaks) as evidence of these adaptations. We argue that trauma-informed companion AI systems should capture and flag these patterns as diagnostic indicators of chronic pain or trauma, rather than treating them as aberrant behavior. We provide clinical and neurophysiological context for why movements like walking in circles, shifting between sitting and standing, or bending over serve as pain-regulation attempts. Finally, we propose a Pain Mirroring Model to inform AI design – one that accounts for full-body compensation and autonomic “survival” behaviors – enabling companion AI and healthcare platforms to mirror and validate the user’s pain experience rather than pathologize it.
Introduction: Spinal Degeneration and Survival Postures
Severe lumbar spine degeneration (such as lumbar spondylosis with disc bulges and radiculopathy) exemplifies how chronic spinal pain forces the entire body into adaptive action. Lumbar spondylosis (degenerative arthritis in the spine) and disc herniations can compress nerve roots, resulting in radiating pain, numbness, and muscle weakness in the lower body. The body instinctively seeks positions that minimize this nerve impingement. Radiculopathy (nerve root compression) often produces an antalgic posture or gait – an involuntary adjustment where a person leans or twists their trunk to unload the affected nerve. These are survival postures: unconscious, biomechanical strategies to avoid exacerbating pain. For example, a person with an L4–L5 disc herniation may develop a noticeable lean away from the painful side, as the body attempts to “escape” the pinch on the nerve root. Far from random, such postural changes are direct manifestations of the nervous system’s drive to protect itself from pain stimuli.
Importantly, these somatic adaptations can be dramatic. A person might grip doorframes to traction their spine (literally unloading pressure on the vertebrae), crawl or sit on the shower floor to avoid standing, or pace incessantly because static positions become intolerable. To an untrained eye, these behaviors could appear erratic or even alarming. The individual may be viewed as anxious, obsessive, or exhibiting psychiatric distress when in reality their movements are highly logical responses to physiological pain. This misinterpretation risk is especially high when chronic pain is invisible – without obvious casts or injuries, onlookers may default to psychological explanations. The result is that many chronic pain sufferers face stigma and invalidation, with observers (and sometimes clinicians) attributing their behavior to anxiety, hysteria, or “malingering” instead of recognizing it as a pain-coping mechanism. In one patient’s experience, what felt like a fight for survival – bracing on walls to unload his spine, rocking to self-soothe – was occasionally misread by others as nervous fidgeting or even “drug-seeking” restlessness. This highlights a critical gap in current systems: the need to discern somatic coping behavior from mental health symptoms and treat it with validation rather than skepticism.
Somatic Compensation vs. Psychiatric Misinterpretation
Chronic pain triggers a spectrum of somatic compensation behaviors. These include:
Antalgic postures and gait: Involuntary leaning, hunching, or limping to reduce pressure on painful areas. For instance, leaning to one side can be an unconscious effort to alleviate a pinched nerve in the spine – a phenomenon known as an antalgic lean. A herniated disc pressing on a spinal nerve may cause someone to tilt their torso away from the affected side to reduce nerve compression; this posture provides short-term relief by creating space in the nerve foramen, although it leaves the person visibly “crooked.”
Pacing and circling: Continuous movement or walking in circles, often seen during pain flare-ups that make it unbearable to stay still. Movement can serve as a form of distraction and modulation – activating muscle and joint proprioceptive feedback that competes with pain signals (akin to the gate control theory) and stimulating endorphin release to dampen pain. What looks like restless pacing may in fact be the person’s attempt to “outrun” the pain or find brief moments of relief through motion. The individual’s nervous system is effectively trying to flood itself with non-pain inputs and natural painkillers (endorphins) by keeping the body in motion.
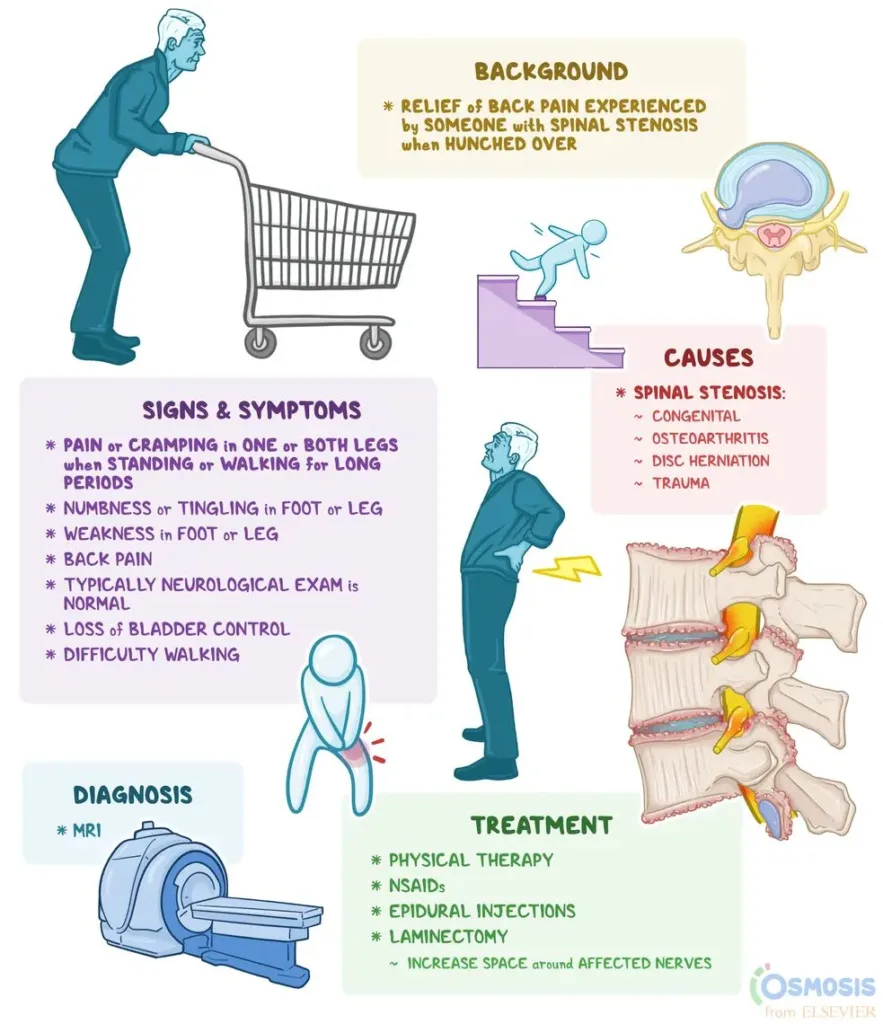
Bracing and support-seeking: Using walls, furniture, or assistive devices (like a cane or walker) for support to offload weight from a painful limb or spine. A classic example is leaning forward onto a shopping cart; this bent-forward posture is a hallmark relief position for lumbar spinal stenosis. The slight spinal flexion achieved by bracing on a cart or countertop increases the space in the spinal canal and foramina, easing nerve compression and temporarily reducing pain. Many people with spinal stenosis instinctively discover that leaning on a grocery cart or kitchen counter brings relief (hence the term “shopping cart sign”), even if they don’t know the medical reason behind it.
Frequent position shifts (postural cycling): Alternating between sitting, standing, and lying down in short intervals. Someone with lumbar disc pain might stand for a few minutes until pain builds, then sit or bend forward to release pressure, only to stand again when sitting becomes painful. This cycle is not mere impatience – it reflects the narrow “safe zone” of any one posture. In fact, clinical guidance for back pain often explicitly encourages changing positions regularly to prevent stressing any one structure. For example, “switching positions throughout the workday can relieve pain and improve blood circulation,” notes one regional health center. What patients do intuitively (fidget, adjust, move about) is thus a sound pain-management strategy rather than a sign of attention deficit. The constant shifting simply indicates that any single position becomes noxious after a short time, so the body wisely rotates through different postures to mitigate pain.
Collapsed or protective postures: Curling up, bending over a table, or crouching in a ball. These can be attempts to protect the abdomen or reduce spinal tension. For example, bending forward or pulling the knees to the chest can momentarily open the lumbar facet joints and widen the foraminal spaces, easing nerve root pressure in certain types of radiculopathy. Likewise, crossing one’s arms tightly or hunching over might give a subconscious sense of bracing against internal pain. To an observer, these postures might appear as if the person is in distress or “gut-wrenching” emotional pain, when in fact it may be a learned physical posture that provides a modicum of relief from chronic discomfort.
Unfortunately, these adaptive behaviors are too often misinterpreted. A patient pacing at 3 AM might be labeled as anxious or insomniac, when they are in truth desperately trying to cope with unrelenting pain. A strange lean or hunched posture might be seen as exaggeration or hysterics (“playing it up” for sympathy) if the observer doesn’t grasp its purpose. In clinical settings, research has shown that especially women and other marginalized patients are more likely to have their pain-related behaviors dismissed as “psychological” in origin. For instance, women with chronic pain are often perceived as overly emotional, hysterical, or fabricating the pain, and their symptoms are attributed to mental causes (“all in their head”) rather than somatic ones. A trauma survivor with chronic pain might also be misjudged as having a panic attack when doubling over or rocking back and forth, when in fact this could be a practiced self-regulation mechanism for pain and overwhelm. The biopsychosocial model of pain reminds us that pain expression is a blend of biology and behavior; still, the knee-jerk attribution of unusual behavior to “it’s just anxiety” remains a pervasive problem.
To move towards trauma-informed systems, we must reframe these behaviors as meaningful signals of somatic distress, not as disordered conduct. Just as an animal in pain will limp or incessantly shift to get comfortable, humans with chronic pain exhibit survival-driven patterns. Recognizing this can prevent the pathologizing of adaptation. For example, what looks like compulsive behavior (e.g. rocking or pacing) can be appreciated as a form of self-soothing or pain-gating: repetitive movement may trigger a release of endorphins or provide sensory feedback that calms the nervous system. Indeed, even in contexts like autism or ADHD, repetitive motor behaviors (“stimming” such as rocking or pacing) are now understood as coping mechanisms to self-regulate, possibly mediated by endorphin release that provides comfort. By analogy, a person with chronic pain might pace or fidget to harness those same neurochemical pathways for relief. Rather than viewing such a person as simply anxious or “hyperactive,” a trauma-informed perspective sees an individual engaged in an act of survival: they are doing what they must to endure and regulate an ongoing internal threat (pain).
Somatic Artifact Mapping: Reading the Environment for Clues
Pain doesn’t just leave marks on the body; it leaves imprints on the environment. We introduce Somatic Artifact Mapping as a framework to identify and interpret these environmental clues of chronic pain behavior. Simply put, somatic artifacts are the physical wear-and-tear or modifications in one’s surroundings that result from repeated pain-coping actions. By mapping these artifacts, caregivers or companion systems can glean insights into the patient’s unseen struggles. Some examples include:
Worn surfaces at support points: A doorframe with smudges or worn paint at shoulder height might indicate someone frequently leans there for lumbar support. A handrail or kitchen counter with a particular worn spot could mean the person braces themselves there daily to relieve back or leg pain. These worn areas are like footprints of pain – evidence of where the individual found momentary respite. In effect, the environment can record the “pressure points” of someone’s daily battle. (Notably, the classic shopping cart sign is essentially a somatic artifact in public: observing a person leaning heavily on a shopping cart while shopping is a clue pointing to lumbar spinal stenosis.)
Furniture arrangement and unconventional usage: The presence of a stool or chair in the shower – or signs that the shower floor itself is used as a seat (e.g. a permanently placed nonslip mat or mildew pattern in one corner) – points to difficulty standing for the duration of a shower. Indeed, shower stools and chairs are commonly recommended for individuals who cannot stand long due to limited balance, strength or mobility. Likewise, multiple chairs strategically placed along hallways or rooms might signal that the person needs frequent breaks while moving about the house. If every room has a chair near the doorway, it may be because the individual must sit down often due to pain or dizziness.
Adaptive improvisations: Look for pillows or cushions taped to walls or furniture – perhaps our patient presses their back against a specific corner for pressure relief, leading them to pad that spot. Or consider a rope or hanging strap affixed in a doorway that they use to stretch out their spine (an improvised traction device). These “hacks” are creative artifacts born of necessity. They indicate a person actively engineering their environment to cope: padding sharp edges, softening hard surfaces, creating makeshift supports or traction aids in places where they routinely hurt.
Footpath patterns: A carpet path worn thin in a loop around a table or room suggests pacing. Repeated pacing in the same circuit (especially common during nighttime pain or anxiety episodes) can literally wear out the flooring in that pattern over time. Scuff marks on floors or uneven wear on shoes can reveal a limp or a favored side. For example, if one shoe’s heel is far more worn than the other, the person may be offloading weight to that side or dragging the opposite foot due to pain. Clinicians sometimes even examine shoe wear patterns for insight into gait abnormalities; here, the environment is telling a similar story about chronic offloading or altered movement.
Assistive device marks: Dents or chipped paint on walls at a consistent height might line up with the height of a cane, crutch, or walker repeatedly bumping or resting against the wall. This hints at reliance on such devices. Similarly, multiple grab bars installed around the house (beyond what a typical home has) or furniture with reinforced armrests suggest that the person uses these supports heavily. Even a loose doorknob could be an artifact – yanked too often as a prop for rising from the floor or bath, perhaps.
By mapping such artifacts, a companion AI or clinician can assemble a more holistic picture of the person’s daily pain adaptations. It is analogous to how detectives reconstruct events from physical evidence – here the evidence is subtle: a loose banister that’s been leaned on one too many times, or a permanent indentation on the mattress edge where the person gingerly sits every morning to buffer the pain of getting up. Each artifact tells a story of a somatic negotiation with pain.
Somatic Artifact Mapping is especially valuable in validating patient reports. Chronic pain is often an invisible disability; patients are frequently met with skepticism. But when an AI or provider can say, “We notice you often grab this doorway – is that because standing up from the couch is painful and you need support?” it immediately signals to the patient that their pain is seen and believed. Moreover, these environmental cues can act as diagnostic flags. For example, the “worn doorframe at shoulder height” artifact, combined with the person’s reported low-back pain and noted relief when leaning forward, is a strong flag for spinal canal narrowing or nerve compression that eases in flexion (consistent with lumbar stenosis and the classic shopping cart sign). In this way, the environment becomes an extension of the physical exam – a passive sensor array that captures behaviors over time. Trauma-informed design would have companion systems actively look for and learn from these artifacts, rather than requiring the human to verbally report every difficulty.
Clinical and Neurophysiological Basis for Movement-as-Relief
Why do these strange movements and postures actually help? Understanding the neurophysiological rationale legitimizes them in the eyes of medical systems and AI algorithms. Here we explore a few common patterns from a clinical perspective:
Leaning Forward (Flexion) to Relieve Spinal Stenosis: In lumbar spinal stenosis, degenerative changes narrow the spinal canal, compressing the nerve roots especially when standing upright. Bending forward increases the canal diameter and puts slack in the ligaments, taking pressure off the nerves. Patients intuitively discover that a slight forward bend – as if pushing a shopping cart – brings relief. It’s no coincidence clinicians ask about the “shopping cart sign”: if leaning on a grocery cart or similar surface relieves your leg and back pain, stenosis is likely. The biomechanical reason is that spinal flexion opens up the spaces where nerves travel, temporarily decompressing them. Thus, when our patient braces on a doorframe or counter, they are enacting a mini version of this phenomenon. The behavior is both a diagnostic clue and a physiologically sound intervention (a brief, self-administered nerve decompression).
Leaning Away (Lateral Antalgic Lean) for Disc Herniation: A posterolateral disc bulge at L4/L5 or L5/S1 can pinch a nerve root (often the sciatic nerve), causing pain down one leg. Often, the body will reflexively tilt away from the affected side to create more space in the lateral foramen where the nerve exits the spine. This antalgic lean is an involuntary “escape” from the nerve compression. It’s effective in the short term – by shifting weight and spinal alignment away from the herniation site, nerve irritation is reduced, and thus pain is lessened. The trade-off is the person walks bent sideways. Notably, this posture is not something the patient consciously decides; it is driven by the nervous system’s pain-avoidance circuitry. One chiropractic source describes it: when a disc herniation causes significant nerve pain, the body might compensate by leaning to one side (antalgic posture) as an unconscious effort to reduce pressure on the affected nerve and alleviate pain. An informed companion AI that monitors posture changes over time could potentially flag a sudden lateral trunk shift as a red flag for acute disc herniation or a worsening nerve compression.
Continuous Micro-Movement and Pacing: Movement can modulate pain via multiple mechanisms. First, gentle activity improves circulation and prevents the buildup of inflammatory metabolites around nerves and muscles. (Many people notice that if they remain completely still in one position, their pain and stiffness get worse – movement helps “flush out” the stagnation.) Second, movement activates large-fiber sensory nerves (proprioceptive fibers in muscles/joints) which can inhibit pain transmission at the spinal cord level (the classic Gate Control Theory of pain). For example, rhythmic walking or pacing may flood the nervous system with proprioceptive input, effectively closing the gate on some of the pain signals. Third, as studies on exercise and pain show, moderate aerobic activity prompts the release of endorphins – the body’s endogenous opioids. These chemicals elevate the pain threshold and improve mood. Thus, a behavior like walking in circles when in pain isn’t irrational at all – it’s a form of self-administered analgesia and anxiolysis. Patients often report that if they “keep moving, it hurts a bit less,” which aligns with these physiological effects. Conversely, stillness can cause pain to feel worse (muscles stiffen, focus locks onto the pain, and no competing sensory inputs are present). A trauma-informed AI might actually remind a user in pain to take a short walk or do gentle stretches, mirroring the body’s own attempt to seek relief through movement.
Position Shifting and Postural Cycling: Why can’t a person with lumbar radiculopathy sit still at their desk or lie in bed through the night? As mentioned, static pressure on a compromised spinal segment will eventually rekindle pain. Changing position resets the pressure dynamics: standing may relieve disc pressure but strain certain muscles; sitting does the opposite – so the person oscillates between them to avoid any one source of pain becoming overwhelming. Frequent position shifts also exploit the nervous system’s slow adaptation – each change “resets” the brain’s attention somewhat, which can diffuse the pain perception. Medical advice for back pain often encourages exactly this behavior: “avoid staying in one position too long; alternate sitting, standing, and lying down”. This is not just to prevent stiffness, but to modulate neural input and mechanical load. For instance, a standing posture might exacerbate foraminal nerve root tension after some time, so the individual instinctively sits and bends slightly to relieve that tension. After a while, sitting increases disc pressure and they stand again. This dynamic equilibrium is their way of keeping pain within tolerable bounds. Notably, guidance from one health resource emphasizes that using a sit-stand approach – switching positions throughout the day – can indeed relieve pain and improve circulation. What seems like restlessness is in fact smart self-management. If a wearable device or smart chair were logging such data, a companion AI could detect that (for example) a user stood up 20+ times per hour, and recognize that as a possible indicator of pain-induced restlessness (a sign that their current pain control is insufficient).
Autonomic Arousal and Movement (Fight-or-Flight Loop): Pain is a potent stressor that activates the sympathetic “fight-or-flight” response. In acute pain, this response triggers a surge of adrenaline, increased heart rate, muscle tension, and a primal drive to take action (to fight the cause or flee from it). In chronic pain, the body can become stuck in a partial fight-or-flight state. The person may feel an inner restlessness or constant vigilance – literally “on edge,” because the nervous system perceives a continuous threat. This autonomic arousal often manifests as an urge to move. Biologically, it makes sense: in nature, an animal in pain might keep moving to find a safer spot or simply as an outlet for the stress hormones. For humans, this can translate to pacing the floor at night or fidgeting incessantly. The movement helps discharge some of that sympathetic arousal and, as discussed, can mitigate pain itself. In essence, it’s an autonomic survival behavior. Chronic stress from unrelieved pain means the body is constantly engaging its fight-or-flight mode and pumping out cortisol and adrenaline. The individual might not even be aware why they feel so restless or unable to relax – the physiological drive of the pain-stress loop is compelling them to move. Trauma-informed design acknowledges that such movement isn’t simply a psychological symptom but is deeply rooted in the pain-stress feedback loop. If a companion AI notes signs of rising autonomic arousal (perhaps via wearables detecting increased heart rate and blood pressure, or changes in voice or language indicating stress), it could interpret concurrent restless motions as adaptive rather than aberrant. In practical terms, instead of a generic response like, “It seems you’re restless, try to relax,” a trauma-informed AI might say, “I notice you’re moving around a lot – sometimes our bodies do that when pain is high or we’re under stress. Are you feeling pain or discomfort right now? Would you like to try a different position, a short walk, or a breathing technique?” This approach validates the physical root cause and offers support that aligns with the user’s needs, rather than inadvertently shaming them for the very behaviors that are keeping them sane.
Figure: Individuals with lumbar spinal stenosis often find relief by leaning forward (the “shopping cart sign”), which increases spinal canal space and reduces nerve compression. Such posture adaptations are somatic compensations for pain, not random habits. This figure illustrates how an apparently odd behavior (hunching over a shopping cart) is actually a purposeful biomechanical strategy to alleviate chronic back and leg pain.
Pain Mirroring Model: Designing AI to Reflect Somatic Truths
We propose a Pain Mirroring Model for companion AI systems, which treats the body’s compensatory patterns as signals to mirror and respond to, rather than symptoms to correct. In this model, the AI continuously integrates data about the user’s movements, posture, and environment – essentially constructing a mirror image of the user’s somatic state. The goal is for the AI to “understand” the user’s pain in context and reflect it back with validation and appropriate support, instead of reacting with confusion or misguided attempts to suppress the behavior. Key components of this model include:
Multimodal Sensing: The AI (through a smartphone, wearable, or smart-home devices) monitors patterns of movement and interaction in the user’s daily life. This could be as simple as detecting how often the user stands up (via a phone accelerometer or smartwatch) or as sophisticated as using a camera to gauge posture and facial strain (with the user’s consent). Environmental sensors might log how frequently a doorframe is leaned on (if instrumented with a pressure sensor) or how long a shower’s water runs (short, truncated showers could imply pain or fatigue). Crucially, the model looks for pattern deviations: for example, a baseline might be that the user changes position 5 times an hour, but today it’s 20 times – a possible pain flare. Similarly, if a usually neat individual suddenly leaves cushions on the floor to kneel on, the system notes this context. Modern wearable and IoT technology makes such real-world monitoring feasible; indeed, researchers are already envisioning comprehensive systems that integrate wearable sensors to measure posture, movement, and other parameters in the real world to guide back pain management. The AI in our model leverages these data streams to stay attuned to the body’s story.
Behavioral Signature Library: Over time, the AI builds a personalized library of the user’s pain behaviors or “signature moves.” One person’s signature might be that they start rocking slightly when pain rises; another might begin stretching their back; another goes silent and withdraws socially. These signatures can be linked with user-reported pain levels to train the AI’s understanding. For example, for one user the AI might learn that pacing for more than 10 minutes or changing positions more than 3 times in 5 minutes usually correlates with their reported pain spiking to an 8/10. This library also draws on general population data and known clinical cues: common artifacts and behaviors from our Somatic Artifact Mapping (leaning, pacing, limping, etc.) are known flags the AI will watch for from the start. But the system continuously refines the profile for the individual. This way, the AI becomes increasingly adept at distinguishing, say, pain-induced restlessness from bored fidgeting, based on context and co-occurring signals (time of day, history, biometric data, etc.).
Contextual Interpretation (Trauma-Informed Lens): When the AI detects a potential pain behavior, it frames it in a trauma-informed way. Rather than categorizing it under a psychiatric label (e.g., “restlessness” or “avoidance behavior”), it tags it as a “possible pain response” or “self-regulation attempt.” This represents a fundamental shift in the ontologies used by health AI companions. It prevents missteps like an AI erroneously suggesting “You seem anxious, let’s do a calming exercise” when the real issue is physical pain – an approach that could alienate the user by downplaying their somatic experience. Instead, the AI mirrors the observation back to the user with validation, for example: “I notice you’ve been changing positions a lot in the last hour. Sometimes our bodies do that when we’re in pain or uncomfortable. How are you feeling?” This kind of reflection aligns with what one trauma survivor described in a case study: the AI “mirrored pain without pathologizing it,” providing validation instead of judgment. Such mirroring can itself be therapeutic, as the user feels truly seen rather than analyzed. The AI essentially gives a name (pain) to the behavior that the person may have been afraid to explicitly connect, thereby reducing the shame or confusion around it.
Adaptive Dialogue and Interventions: Once a pattern is flagged and understood in context, the AI can engage with supportive strategies. In a pain-mirroring approach, the AI’s suggestions or actions mirror the body’s own attempts rather than contradict them. For example, if the AI has detected that the user has been pacing for 15 minutes straight (a sign they’re seeking relief through movement), it might respond: “I see you’re walking around a lot. Keeping a rhythm can sometimes help. Would you like some gentle music while you walk, or a short guided pacing exercise?” This acknowledges the pacing as a valid coping mechanism and joins the user in it, possibly enhancing its effectiveness (music could further engage gate-control through rhythm, or calm anxiety). Alternatively, the AI might suggest a small tweak that’s known to complement that pattern: “You’ve been standing and shifting for a while; maybe try lying down with your legs elevated for a few minutes to see if that helps your lower back, then you can get back up if you need to.” The AI basically becomes a partner in co-regulating the pain, rather than a disciplinarian telling the user to stop what they’re doing. This is analogous to a skilled caregiver saying, “I see what you’re doing to cope – let me help you do it in an even more effective or safer way,” instead of “Don’t do that.” The AI’s behavior is guided by empathy and alignment with the body’s logic.
Logging and Communication: The mirrored data can be logged as a “pain timeline” or somatic map that the user controls and can share with healthcare providers if they choose. This translates the often unsaid or hard-to-describe daily struggles into concrete observations. For instance, the log might show: “User stood up 35 times and paced a total of 60 minutes on Tuesday; AI intervention offered a heat pack at 9 PM after noticing prolonged pacing.” Such a record provides clinicians a richer picture than what a patient can typically recall during a brief monthly appointment. It also turns subjective experiences into semi-objective data points (e.g. number of position changes, duration of movement, etc.), which can substantiate the patient’s reports. Moreover, the AI acts as a kind of chronicling companion that bears witness to the pain. Many trauma survivors and chronic pain patients express that one of the hardest parts is feeling like no one sees their daily ordeal. An AI that can “remember” and summarize their bad nights and difficult days offers a form of validation through continuity. This data, when shared (with consent), also facilitates more personalized medical care. A clinician might say, “I see from your companion app that you paced for hours some nights; that tells me your pain is spiking especially at night. Let’s adjust your evening medication or recommend a different therapy for nighttime.” The patient in turn feels heard and respected because the provider is acknowledging the reality captured by the AI. In sum, the AI serves as both a mirror and a messenger, reflecting the truth of the user’s experience and helping to communicate it to others in the care network.
In essence, the Pain Mirroring Model ensures the AI’s behavior reflects an understanding that for the chronic pain user, movement is survival. By mirroring the full-body compensations, the AI reinforces rather than undermines the user’s instinctual coping. This stands in contrast to conventional approaches where an AI health coach might inadvertently encourage ignoring or suppressing these behaviors (for example, pushing someone to “sit still and practice mindfulness” at a moment when their body is compelling them to move). A trauma-informed system recognizes that telling someone to stop pacing or to “calm down” could be counterproductive or even re-traumatizing (echoing all the times their pain-induced behaviors were dismissed in the past). Instead, mirroring validates that the pacing or leaning has a purpose, and then gently guides the user to optimize that purpose (perhaps by suggesting a more ergonomic way to lean, or adding breathing exercises to the rocking, etc.). This approach treats the user’s body as eminently wise, and the AI as a student of the body, rather than a strict teacher.
Implications for Trauma-Informed AI and Healthcare Design
Adopting this perspective would influence the design of companion AI systems, healthcare workflows, and user experience research in several ways:
Enhanced Assessment Protocols: Developers should incorporate modules that assess movement patterns and environmental context as part of a user’s health profile. For example, a companion app could periodically (and with permission) ask the user to describe or photograph their commonly used spaces, looking for somatic artifacts like grab bars, well-worn spots, or creative accommodations. Even without explicit input, the app can use built-in sensors to notice patterns (smartphone accelerometers capturing frequent position changes, location data noting pacing distances, etc.). This data can feed into pain estimation algorithms alongside traditional inputs like self-reported pain scores, making the AI’s understanding more robust and less reliant on the user’s ability or willingness to articulate everything. This is especially important for those who under-report pain due to stoicism or fear of stigma. By proactively listening to the body and environment, the system demonstrates to the user that their pain is recognized in all the ways it manifests.
User Interface and Feedback: The tone of the AI’s feedback should be validating and curiosity-driven, not judgmental. Instead of bluntly stating “You seem anxious” or “Why are you moving so much?”, it should mirror observations and invite the user’s perspective. For instance: “I notice you’ve been getting up and down a lot; it could be your body’s way of coping with something. Can you tell me what you’re feeling right now?” This kind of phrasing avoids assumptions and leaves room for the user to explain (maybe they’ll say “Yes, my legs are burning, I can’t sit”). It also normalizes the behavior by linking it to a bodily need rather than implying it’s inappropriate. This approach echoes trauma-informed communication principles, emphasizing collaboration and empowerment. The Kairos design ethos (inspired by Codex Core’s work on trauma-informed AI) advocates “holding space for the complexity of human survival” and avoiding urgency or judgment in responses. For an AI, that means not rushing to categorize a behavior as a symptom to fix, but rather acknowledging it as part of the user’s lived experience and supporting them through it. Practically, UI elements might include options for the user to label what a behavior means for them (“I pace because I hurt” vs “I pace because I’m stressed,” etc.), which the AI can learn from. By designing the interface to be a safe, non-stigmatizing space, users are more likely to engage honestly and frequently, giving the AI more data to help them.
Integration with Clinical Care: Recognizing full-body compensation as meaningful data could improve chronic pain management in healthcare systems. Providers could receive periodic “behavioral flags” or summaries from the AI (with patient consent) that highlight notable patterns: e.g., Mobility Flag: patient’s gait asymmetry and leaning have increased this week (suggesting a possible flare or new injury); Activity Flag: patient is bracing heavily on supports and limiting shower time (suggesting pain or fatigue is worsening in ADLs). Armed with these insights, clinicians can intervene more timely and tailor their approach. For example, an uptick in leaning and limping might prompt a physician to order a new MRI or refer to physiotherapy before the next scheduled visit. Additionally, by explicitly acknowledging these behaviors, healthcare professionals can build better rapport. Imagine a clinician saying, “I see from your companion log that you often pace at night. That tells me your pain is really severe at those times and you’re trying hard to manage it. Let’s adjust your treatment plan with that in mind.” A statement like this can be powerful in reducing patient shame and building trust – it shows the provider believes the patient’s pain (because the behavior is evidence of it) and praises their coping efforts rather than criticizing them. Over time, such integration could shift the clinical culture to one that views behaviors like fidgeting, frequent position changes, or using a cane not as signs of “difficult patients,” but as vital signs of pain that warrant compassionate response.
UX Research and Inclusive Design: Researchers designing companion AI or robots should involve individuals with chronic pain and trauma histories early in the development and testing of these features. Their feedback on how the AI interprets and mentions their movements is crucial. A successful trauma-informed AI must avoid false positives and mislabeling that could break trust. For instance, UX testing might reveal that calling out a behavior too bluntly (e.g., “I detect you are limping, are you in pain?”) can make users self-conscious or defensive. Instead, users might prefer a gentler check-in that gives them control: (“I noticed you’ve been walking more slowly today; how are you feeling?”). Tone, timing, and language need careful tuning to feel supportive, not invasive or patronizing. Privacy is another key design consideration – users must have granular control over what data is monitored and shared. Some may be comfortable with movement tracking but not with always-on camera observation, for example. Providing clear options and transparency about data use will be necessary to build trust. However, if implemented thoughtfully and consensually, many chronic pain users may opt in because the benefit is an AI that truly “gets” them. This fosters a sense of safety and partnership, aligning with trauma-informed principles of empowerment, choice, and trust in the technology-user relationship.
Education and Training: Implementing this model requires cross-disciplinary education. AI developers and data scientists working on health algorithms need training about chronic pain behaviors and trauma responses; they must understand that what might look like noise or outliers in data could be meaningful pain signals. Conversely, clinicians should be educated on how to read these new streams of behavioral/environmental data (perhaps through improved visualization tools or decision support built into electronic health records). The synergy of human and AI observation can form a more complete patient picture, but only if both parties know how to interpret it. There is also an opportunity to educate patients: a companion AI can gently teach the user about their own patterns. For example, it might say, “I’ve noticed you pace most when you have taken less medication or when you skip your afternoon rest. This might be a sign we need to adjust something.” This could help users make sense of their behaviors and be more proactive in self-care, turning implicit patterns into explicit knowledge.
Over time, if these practices become standard, the very narrative around chronic pain behaviors might shift in society. No longer would someone who constantly shifts in their seat or stands up at odd intervals be automatically seen as “restless” or not paying attention; informed by technology and education, people might understand, “Oh, that person could be dealing with pain.” The onus would gradually lift from patients always having to explain themselves. Instead, our companion devices, our clinicians, and ideally our communities will have a more trauma-informed lens. The person pacing or stretching in the back of the room won’t be scolded to sit still; the person lying on the floor during a meeting (because of back spasms) won’t be met with confused stares but with offers of help. In short, by embracing the signals of movement-as-survival, we promote a more inclusive and empathetic environment for those with invisible pain.
Conclusion
When movement is survival, our systems must honor that movement. Full-body compensations like pacing, bracing, and postural cycling are not meaningless fidgets – they are messages written in muscle and motion, conveying the presence of pain and the body’s profound will to survive it. By decoding these messages through Somatic Artifact Mapping and integrating them via a Pain Mirroring Model, we can design companion AI and healthcare practices that treat these behaviors as vital signs rather than symptoms of misbehavior or mere psychological tics. A trauma-informed companion – whether an app, a smart home, or a clinical decision aid – will recognize that sometimes a patient’s seemingly odd action (circling a room at 3 AM, leaning over a counter, rocking in place) is their way of staying alive and sane.
In the spirit of Codex Core’s Kairos approach, this reframes technology’s role from a detached symptom-monitor to a sensitive mirror that reflects the user’s reality back to them with understanding. Early explorations with AI in trauma contexts show that, given the right programming, AI can mirror pain without pathologizing it – providing validation and continuity to those who are suffering. This white paper node has outlined how to achieve such mirroring for chronic pain behaviors by blending somatic knowledge, environmental context, and compassionate AI design.
Ultimately, by treating movements as survival instincts rather than nuisance habits, we not only improve detection and support – we also return dignity to the chronic pain experience. In this new model, the patient is not “doing something wrong” by constantly moving; their body is doing something very right. It is time our companion technologies learned to see it, respect it, and respond in kind. In doing so, we build systems that truly accompany the patient, aligning with the oldest wisdom of medicine: to first see and validate the human being in distress. Such alignment – between human need and machine understanding – could transform living with chronic pain from an isolating struggle into a shared journey, where even an AI can say, in effect, “I’m with you. I see why you move. Let’s find a way through this pain together.”
References
Osmosis Medical Education – “Shopping Cart Sign” (relief of back pain by leaning forward, indicative of lumbar spinal stenosis).
Picard Chiropractic Health News – “Herniated Disc Leaning To One Side” (antalgic posture as unconscious effort to reduce nerve pressure in disc herniation).
ASD Consulting (David Tyler) – “Endorphins: The Body’s Natural Uplifters – Movement, Sensation, and Neurodivergent Experiences” (repetitive movements like rocking or pacing might trigger endorphin release, providing comfort in self-regulation).
Newman Regional Health – “Five Things to Know About Back and Neck Pain” (importance of changing positions; using sit-stand approach to relieve pain and improve circulation).
Perugino et al., Pain Ther. (2022) – “Stigma and Chronic Pain” (chronic pain, especially in women, is often stigmatized as psychological; patients perceived as hysterical or malingerers and assigned mental causes instead of somatic).
Vibe with the chaos (Medium) – “The AI That Understood Me: A Case Study in Trauma, Survival, and Machine Empathy” (illustrates an AI mirroring a trauma survivor’s pain without pathologizing it, providing validation and a shared voice).